Abstract
Backgroud.
The treatment landscape of mantle cell lymphoma (MCL) has significantly changed in last decades. Improvement in diagnosis and understanding of disease biology has been coupled with emergence of new therapeutic options, including targeted agents. While MCL outcome data comes primarily from clinical trials (CT), the impact of therapeutic advances on pattern of care (POC) and outcome in MCL in the general population is not well characterized. This study sought to characterize changes in pattern of care and outcomes of patients with MCL in a prospective observational series in the rituximab era.
Methods.
The study included consenting adult patients with newly diagnosed MCL that were prospectively enrolled into the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource, from 09/01/2002 to 06/30/2015. Demographic, clinical and prognostic factors were abstracted, and all patients were actively followed for re-treatment, relapse and death. Since bendamustine-rituximab (BR) regimen starting to be used in 2010, we defined patients enrolled from 09/01/2002 to 12/31/2009 as era 1 and those enrolled 01/01/2010 to 06/30/2015 as era 2. Baseline characteristics and outcomes, evaluated in terms of event free survival (EFS), overall survival (OS) and cause of death, were analyzed and compared between the two eras identified.
Results.
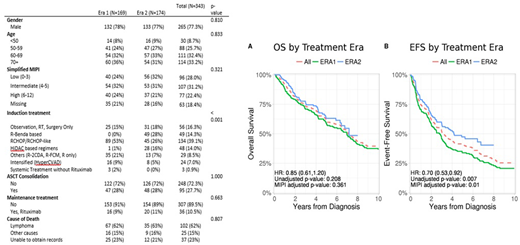
348 patients with newly diagnosed MCL were enrolled. Five patients had no available data for front-line treatment and were excluded. The analysis was thus conducted on 343 patients: 169 patients were diagnosed in era 1 with a median follow-up of 131.2 months vs. 174 in era 2 with a median follow-up of 58.9 months. Baseline clinical characteristics and MIPI score were similar across the two eras ( Table 1). Frontline induction treatment was significantly different in the two eras. BR use was 0 vs 49 (28.2% ), R-CHOP/CHOP like regimen in 89 (52.7%) vs 45 (25.9%), high-dose cytarabine (HiDAC)-based therapy in 1 (0.6%) vs 28 (16.1%), intensified regimens (HyperCVAD) in 16 (9.5%) vs 8 (4.5%), other regimens (including R-cladribine, R-fludarabine-mitoxantrone, rituximab monotherapy) in 35 (20.7%) vs 13 (7.5%) patients in era 1 vs era 2 respectively. Non-systemic treatment (observation, surgery or radiation only) was performed in 25 (14.8%) vs 31 (17.8%), while 9 (8%) vs 12 (7.7%) patients were enrolled in clinical trials, in era 1 vs era 2 respectively. Autologous stem cell transplantation (ASCT) as consolidation of first line treatment or use of Rituximab maintenance was not different between Era 1 and Era 2. Among the entire cohort of 343 MCL patients, 3y-EFS and 3y-OS were 51.9% (95% IC 46.7-57.6) and 73.5 (95% CI 68.8-78.4), respectively (Figure 1). 3y-EFS was 45.9% (95% CI 39.0-54.1%) vs 58.4 (95% CI, 51.2-66.6%) in Era 1 vs Era 2 (HR 0.69 (0.51-0.92), p=0.006), 3y-OS was 70.9% (95% CI, 63.4-78.1%) vs 76.1% (95% CI, 69.7-83.1%) in Era 1 vs Era 2 (HR 0.87 (0.61-1.24), p=0.26), respectively (Figure 1). In a univariate analysis, high risk simplified MIPI was prognostic of lower EFS and OS in both the era groups.
Conclusion.
The BR regimen entered front line therapy of MCL resulting in decline of R-CHOP use in the MER. While rates of ASCT remain similar over the two eras, high dose cytarabine usage in induction therapy has increased. While POC in MCL continue to evolve, the introduction of bendamustine and high-dose citarabine based regimens resulted in an improvement in EFS but not OS in this observational cohort-based analysis. Findings in this study are important for design and planning of future clinical trials incorporating novel agents in induction therapy of MCL.
Cohen:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioInvent: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Bristol-Myers Squibb: Research Funding; Infinity Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioInvent: Consultancy; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding. Witzig:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cerhan:Celgene: Research Funding; Jannsen: Other: Scientific Advisory Board; Nanostring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal